May we send our best wishes and seasonal greetings to all of our Customers and Business Partners, with the hope the coming year 2026 bring you and your loved ones good health, happiness and many joyful moments.
Category: Cosmetics and supplements
Video: The J.S. Hamilton Laboratory in Gdynia is now completely functional.
We are delighted to announce you that the J.S. Hamilton Laboratory in Gdynia is fully operational and offers a comprehensive range of services related to the testing of food and dietary supplements.
We are happy to show you a video that shows what we do every day, how we organize our analytical processes, and how our specialists work.
Our 745 square meter facility houses labs conducting research on nutritional value, vitamins and dioxins, classical, physico-chemical and instrumental analyses.
We encourage you to view the video!
Advanced wrinkle analysis using PRIMOS CR – project implementation successfully completed!
We are pleased to announce the introduction of a new diagnostic service utilizing the PRIMOS CR system. PRIMOS is a state-of-the-art clinical research tool designed for the evaluation of skin microstructure and advanced wrinkle assessment. It provides precise quantitative results for wrinkle depth, length, volume, number, and surface area within the measured zone.
How does PRIMOS CR work?
PRIMOS CR operates using fringe projection technology, also known as phase-shifting profilometry. In this method, a precisely modulated fringe pattern is projected onto the skin surface. As the light pattern becomes distorted by the skin’s microtopography, the system captures these deformations and reconstructs a highly accurate 3D topography of the skin.
Thanks to the implementation of phase-shifting interferometry, PRIMOS CR achieves exceptionally high axial resolution (up to 0.1 μm) and lateral resolution, making it ideal for analyzing fine skin structures and subtle surface changes.
The development of dedicated analytical scripts and the integration of automated data processing through the VECTRA analysis software.
What parameters does PRMOS CR test?
- Standardized wrinkle assessment (depth, length, volume),
- Skin roughness analysis based on parameters: Ra, Rz, Rt,
- Time-lapse comparison of changes (monitoring treatment effectiveness).
These automated scripts also allow for in-vivo comparative studies (before and after treatment), as well as the evaluation of cosmetic product efficacy. This makes PRIMOS CR an invaluable tool in both clinical practice and R&D laboratories.
Book a consultation with our expert Oliwia Kalinowska and tailor a study protocol according to the latest industry standards. Please contact us at cosm@jsh.com.pl.
What do the images generated by PRMOS CR look like?
- Eye wrinkles:
| Subject’s no. | X. |
| Before (D0) | 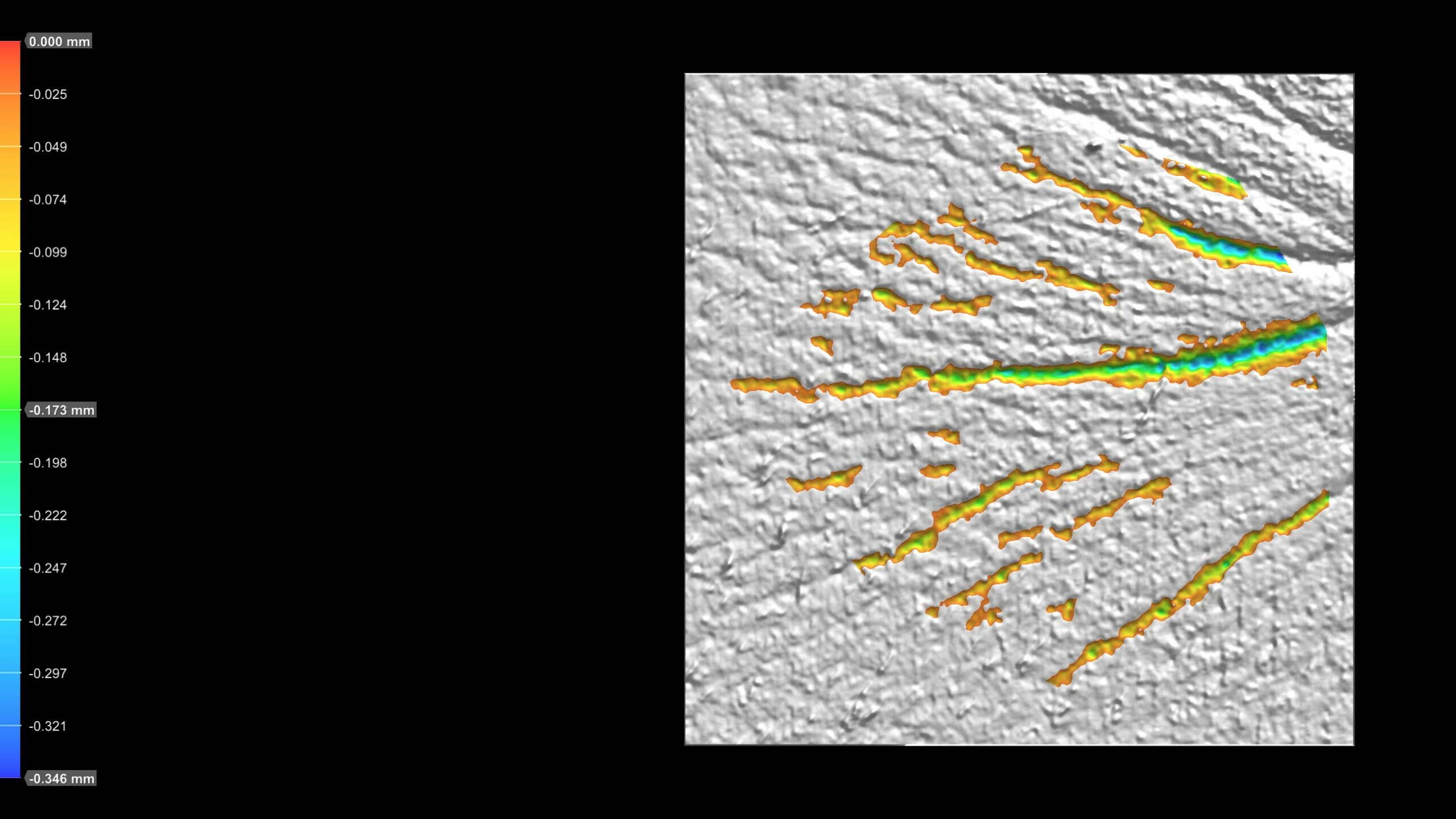 |
| After X days (DX) | 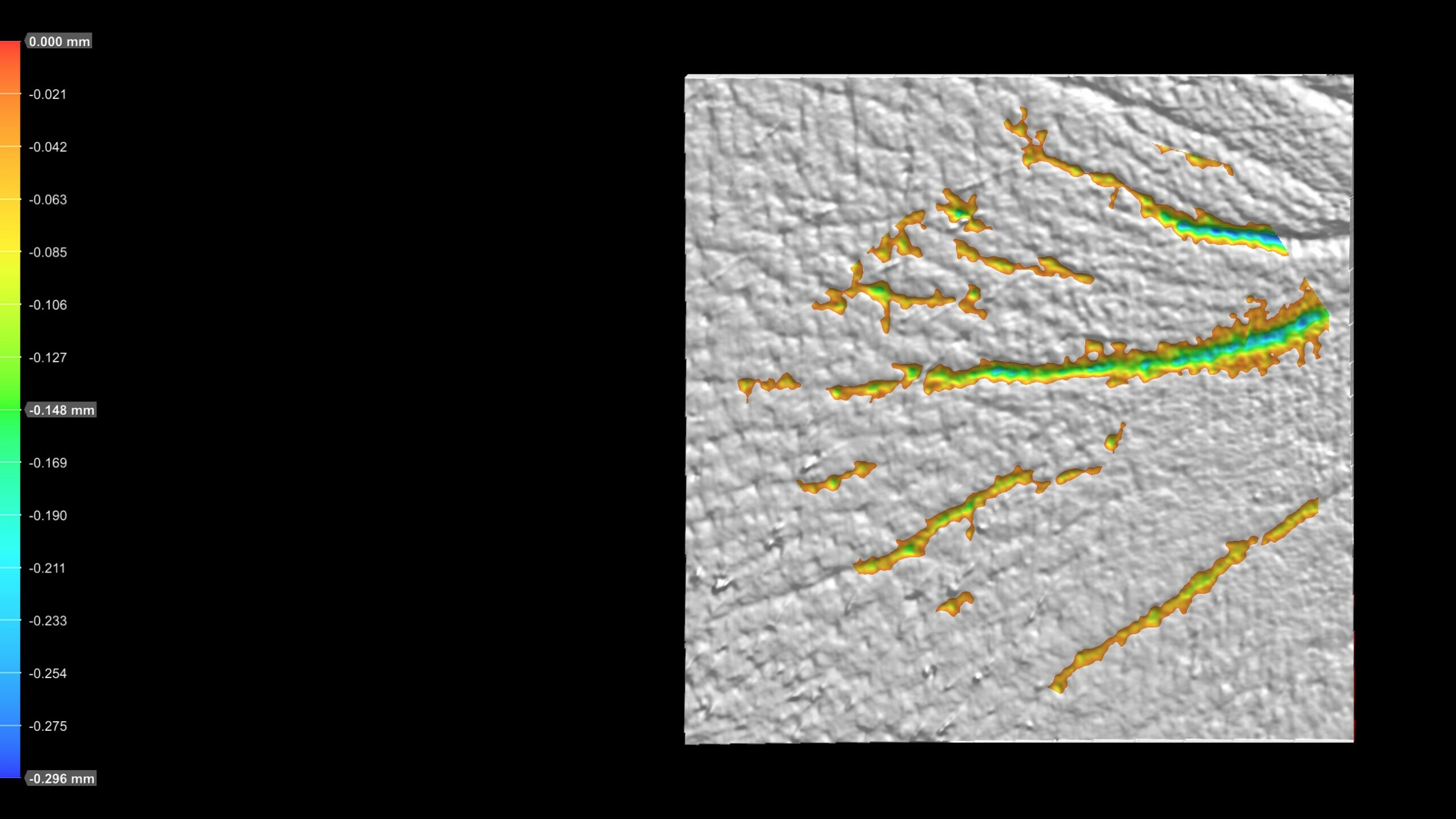 |
| Subject’s no. | Before (D0) | After X days (DX) |
|
X. |
 |
 |
- Forehead wrinkles:
| Subject’s no. | X. |
| Before (D0) |  |
| After X days (DX) |  |
| Subject’s no. | Before (D0) | After X days (DX) |
|
X. |
 |
 |
Our qualified team will provide a suitably selected panel of subjects in accordance with the Client’s requirements.
Ask our experts about research offers using PRIMOS CR.
September in the cosmetology laboratory – news, tests, and important information for the cosmetics industry
Cosmetics tests is constantly evolving, and with it, the importance of products that have laboratory-confirmed efficacy and safety is growing. September in our cosmetology laboratory is a month full of news and important events – both for cosmetics manufacturers and for anyone who wants to develop their portfolio in accordance with the highest standards. Check out what’s new and how we can support you in the cosmetics testing process.
HOW TO PREPARE DOCUMENTS FOR COSMETIC PRODUCT TESTING?
In order to begin application, stability, or microbiological testing without unnecessary delays, it is important to prepare the documentation properly. Depending on the type of order, different sets of documents are required. Remember, the sooner you provide a complete set of information, the sooner we can begin testing your cosmetic product.
| Dermatological Testing | Application & Instrumental Testing | UV Testing |
*Not required for products with low microbiological risk. |
|
|
PROFICIENCY TESTING AND “EXCELLENT PERFORMANCE” RESULT
Our Cosmetics Microbiology Laboratory in Tychy took part in proficiency testing for the preservation test (Challenge test), organized by the German company DRRR – an international leader in this field.
We are proud to announce that we have achieved an “Excellent Performance” result, the highest rating confirming our competence.
For our customers, this is a guarantee that the preservation tests we perform are carried out in accordance with the highest quality standards and with complete reliability.
PAO – PERIOD AFTER OPENING. WHAT DOES IT MEAN AND WHY IS IT IMPORTANT?
Cosmetic packaging often features a symbol of an open jar with a label, e.g., 6M, 12M, 24M. This is PAO (Period After Opening), i.e., the shelf life of the product after opening.
Why is PAO testing crucial?
- It determines how long a cosmetic product is safe to use.
- It indicates how long a product retains its properties after opening.
- It helps to avoid the risk of microorganism growth and deterioration of the formulation.
We conduct PAO testing based on physicochemical and microbiological stability tests under conditions that reflect everyday use.
It is worth remembering that cosmetics with a shelf life of more than 30 months do not need to have an expiry date – the PAO symbol is sufficient.
Would you like to check the shelf life of your product? Contact our expert by filling out the contact form or sending a message to: cosm@jsh.com.pl
CONFIRMATION OF MARKETING CLAIMS – IN VIVO TESTS
Claims such as “moisturizes the skin,” “reduces wrinkles,” or “restores radiance” require confirmation by reliable test results. In our laboratory, we conduct in vivo tests that provide a solid basis for marketing communication.
Why choose our laboratory?
- We conduct tests on appropriately selected groups of volunteers.
- We ensure the safety and comfort of participants.
- We use modern assessment methods in accordance with current standards.
Thanks to the results obtained, your marketing messages will be credible and competitive on the market.
Ask about our range of application and usability tests that support the positioning of cosmetic products.
LET’S MEET AT THE COSMETORIUM TRADE FAIR IN BARCELONA
On October 22-23, 2025, we will be present at the Cosmetorium trade fair in Barcelona – the most important event in the cosmetics industry in Spain. This is an excellent opportunity to discuss cooperation opportunities and learn how our cosmetics testing supports product development:
- we confirm marketing claims,
- we verify the safety of cosmetics,
- we evaluate the effectiveness of formulations.
If you are planning to launch a new product, please contact us and arrange a meeting during the fair. Anna Pawlus, our Account Manager, will be present at the fair – apawlus@jsh.com.pl.
September in our laboratory is a time full of events and important information for the cosmetics industry. Participation in the Cosmetorium trade fair, excellent proficiency test results, PAO tests, and in vivo studies—we do all this to support cosmetics manufacturers in creating safe and effective products.
If you are looking for a partner for cosmetic testing, contact us and find out how we can help you develop your brand.
If you have any questions or concerns, J.S. Hamilton’s experts are at your disposal.
Contact your account manager: cosm@jsh.com.pl
J.S. Hamilton Receives CIR Accreditation – Supporting Innovation with Recognized R&D Excellence
We are excited and proud to share that J.S Hamilton has officially received the Crédit d’Impôt Recherche (CIR) accreditation—a noteworthy achievement for our Microbiology Division and other departments. This recognition reinforces our unwavering dedication to innovation and client collaboration. With CIR accreditation, we’re not only deepening our partnerships but also creating new opportunities for clients to access valuable financial incentives.
What Is CIR Accreditation?
The Crédit d’Impôt Recherche (CIR) is a French tax credit designed to support companies investing in research and development. Both French and international firms operating in France can benefit from a tax reduction of up to 30% on eligible R&D expenditures, particularly in the creation of new products.
What Does This Mean for Our Clients?
▶️Lower R&D Expenses:
Partnering with us allows clients to receive a tax credit of up to 30% on qualified research costs—making innovation more accessible and cost-effective.
️▶️Reliable, Compliant R&D Investment:
Our structured methodologies and robust processes ensure your R&D efforts are well-documented and compliant.
▶️ Trusted Collaboration & Technical Expertise:
Clients gain access to a team of seasoned research professionals, bringing valuable insights and strategic support. Our CIR status affirms the quality and credibility of our innovation capabilities.
▶️ A Legacy of Industrial Excellence at Your Service:
Backed by over 70 years of experience in laboratory testing.
If you are interested in cooperating or would like to find out more, please contact us via contact form.
Happy Easter!
On the occasion of Easter, we would like to extend our best wishes to all our customers and business partners. May this time be full of peace, joy and inspiration for new challenges!

Merry Christmas and a Happy New Year!
May we send best wishes and seasonal greeting to all our Customers and Business Partners, together with prosperity and good health for everyone and their families in 2025.

New scope of accreditation in the Dermatology and Stability section!
A great success in the Dermatology and Stability section. We are the first laboratory in Poland to be accredited for dermatological testing!
The 3-day audit by the Polish Accreditation Centre was a complete success – no nonconformities were found!
We have completed the implementation project to extend the scope of AB 079 accreditation, which includes dermatological methods:
- PB-561 Presence of allergic reaction / contact eczema, in vivo skin irritation method, semi-open and closed test and
- PB-562 Presence of allergic reaction / contact eczema, in vivo skin irritation method, open test.
The accreditation audit was conducted at the Cosmetology Laboratory by an authorized auditor of the Polish Centre for Accreditation, confirming compliance with the requirements of the ISO 17025 standard and the high competence, reliability and impartiality of the JS Hamilton team.
Your products deserve the highest quality service!
If you have any questions or concerns, J.S. Hamilton Experts remain at your disposal.
Increase knowledge about the safety of your supplements
Product awareness is growing among consumers. Recipients are increasingly verifying compositions, seeking information on studies conducted, and choosing products most suited to their needs.
Trends in healthy eating and supporting the body with dietary supplements have forced manufacturers and distributors to pay more attention to the quality of pharmaceuticals and confirm it with independent research. Although dietary supplements are not drugs and are not subject to the same stringent regulations, growing consumer awareness is challenging them to subject their products to the best possible scrutiny.
The choice of remedies and their availability is virtually unlimited. Widespread advertising, however, is not an adequate incentive for informed consumers. Information about product efficacy and safety also plays an important role. Recipients increasingly pay attention to whether a product has been tested by accredited testing laboratories. Keeping in mind the growing knowledge of consumers, more and more supplements are checked by testing methods based on polish and international standards (PN & ISO).
At the J.S. Hamilton laboratory, we test, among other things:
- microbiological safety,
- pyrrolizidine and tropane alkaloids,
- pesticide residues,
- heavy metal content,
- vitamin and mineral content,
- stability of the dietary supplement,
- various types of physicochemical parameters,
- as well as many other parameters.
It is worth noting that dietary supplements of adequate quality can be a valuable addition to a balanced diet, but as long as they are not properly controlled, one cannot be sure of the quality of the available products. By raising awareness about the safety of a product, through research confirming its composition, it is easiest to build trust in products.
At J.S. Hamilton, we provide the highest quality of customer care and closely tailored offerings.
If you have any questions or concerns, J.S. Hamilton Experts are at your service.
Commission Delegated Regulation (EU) on new hazard classes
The new COMMISSION DELEGATED REGULATION (EU) 2023/707 of 19 December 2022 amending Regulation (EC) No 1272/2008 as regards hazard classes and criteria for the classification, labelling and packaging of substances and mixtures was published.
This regulation introduces new hazard classes for the following substances and mixtures:
- having endocrine disrupting (ED) for human health as well as environment,
- persistent, bioaccumulative and toxic (PBT), very persistent and very bioaccumulative (vPvB),
- persistent, mobile and toxic (PMT), very persistent and very mobile (vPvM).
Definitions, classification criteria for substances and mixtures, and label elements were established.
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
If you have any questions or concerns, the J.S. Hamilton Experts remain at your disposal.
Pyrrolizidine alkaloids
The Commission Regulation (EU) No. 2020/2040 of 11 December 2020 has applied since 1 July 2020. It regulates the maximum levels of pyrrolizidine alkaloids in food and dietary supplements.
The limits refer to, among others, herbal dietary supplements, teas, herbs, and herbal teas.
Legally, the foodstuffs listed in the Annex to the Regulation placed on the market before 1 July may be marketed until 31 December 2023. After 1 July 2022, each product covered by the Regulation should obligatorily meet the legal requirements in this area.
On 30 June 2022, the J.S. Hamilton Laboratory successfully passed the audit of the Polish Center for Accreditation, extending the accreditation with the determination of pyrrolizidine alkaloids.
Currently, we can perform this testing in accordance with the EU Commission Regulation No. 2020/2040 of 11 December 2020 and with Ph. Eur. 10.6 chapter 2.8.26.
We look forward to doing business with you!
Perform dermatological tests and use our sign

New service of wrinkles analysis
We are pleased to inform you that our cosmetology laboratory implemented a new service of skin microstructure and wrinkles analysis performed by Primos 3D Lite equipment. Three dimensional measurement, recording and evaluation of the surface structure of skin provides a significant step forward in supporting studies for cosmetic treatments. Results can be presented in the following way:
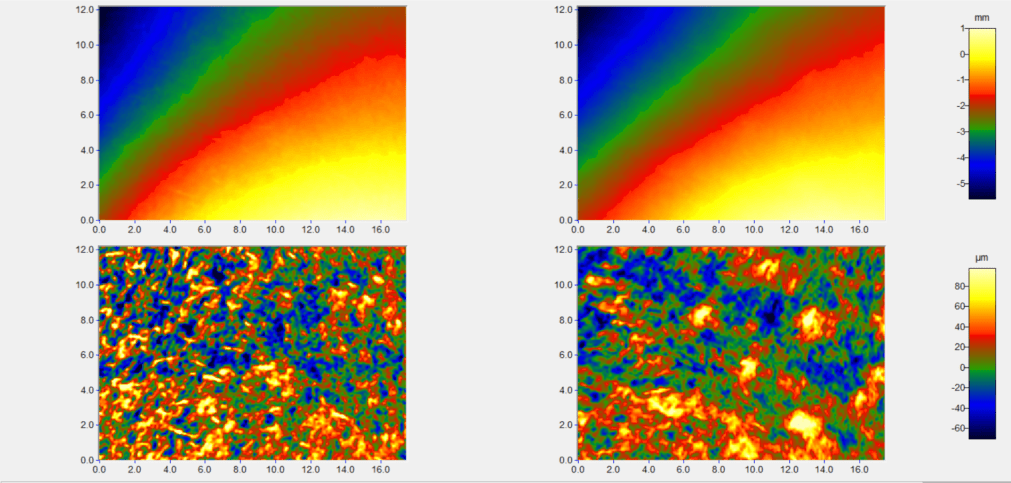
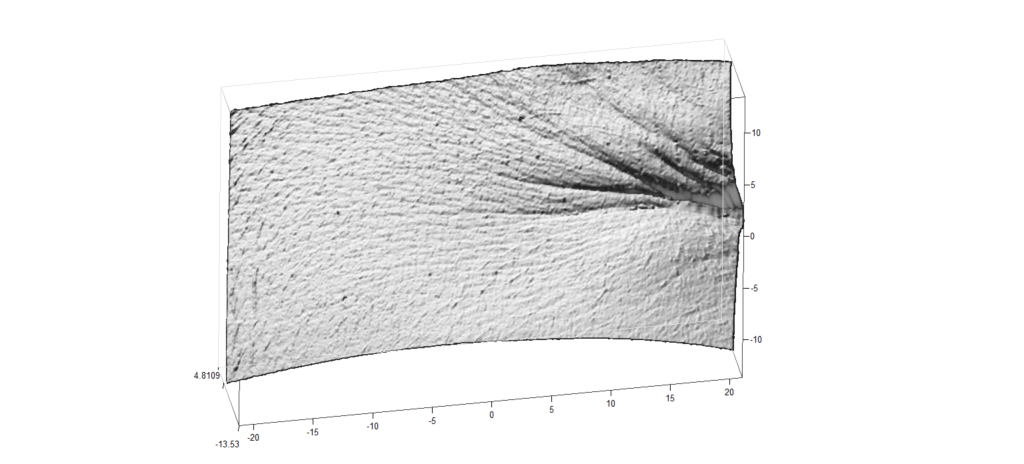
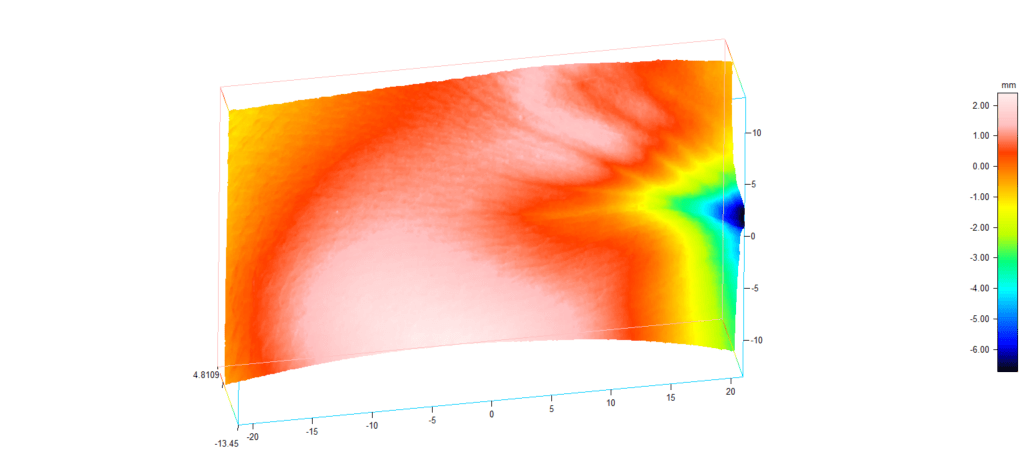
For more information please contact us under phone number +48 58 766 99 74 or send an inquiry to cosm@jsh.com.pl
USFDA inspection
In July 2016 J.S. Hamilton Poland has passed USFDA inspection, on the study of medicinal products and starting materials. As a result of the positive assessment by USFDA – considered to be one of the most demanding, prestigious and influential agencies in the pharmaceutical industry we have receive USFDA approval without observations documented on Form 483.

